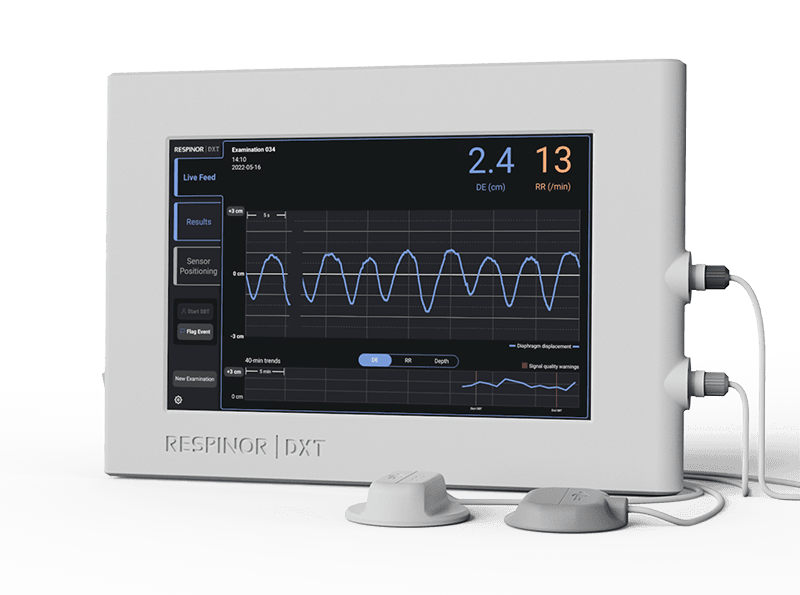
Respinor DXT
DXT, or Diaphragm Excursion Technology, is a ground-breaking non-invasive medical device developed by RESPINOR that utilizes ultrasound for operator-independent, continuous monitoring of the diaphragm, the main breathing muscle, in critically ill patients.
This technology aids in the early detection of diaphragm dysfunction and enhances clinical decision-making in critical care settings.
This technology aids in the early detection of diaphragm dysfunction and enhances clinical decision-making in critical care settings.